History

Encounter with Diabetes Stem Cells
In October 1999, Dr. Hideto Kojima planned a gene therapy targeting the liver at Baylor College of Medicine, based on mechanisms of pancreatic islet development. Using transcription factors involved in pancreatic development (Pdx-1, Ngn3, NeuroD1), islets were regenerated in the liver of mice, aiming for a cure for diabetes.

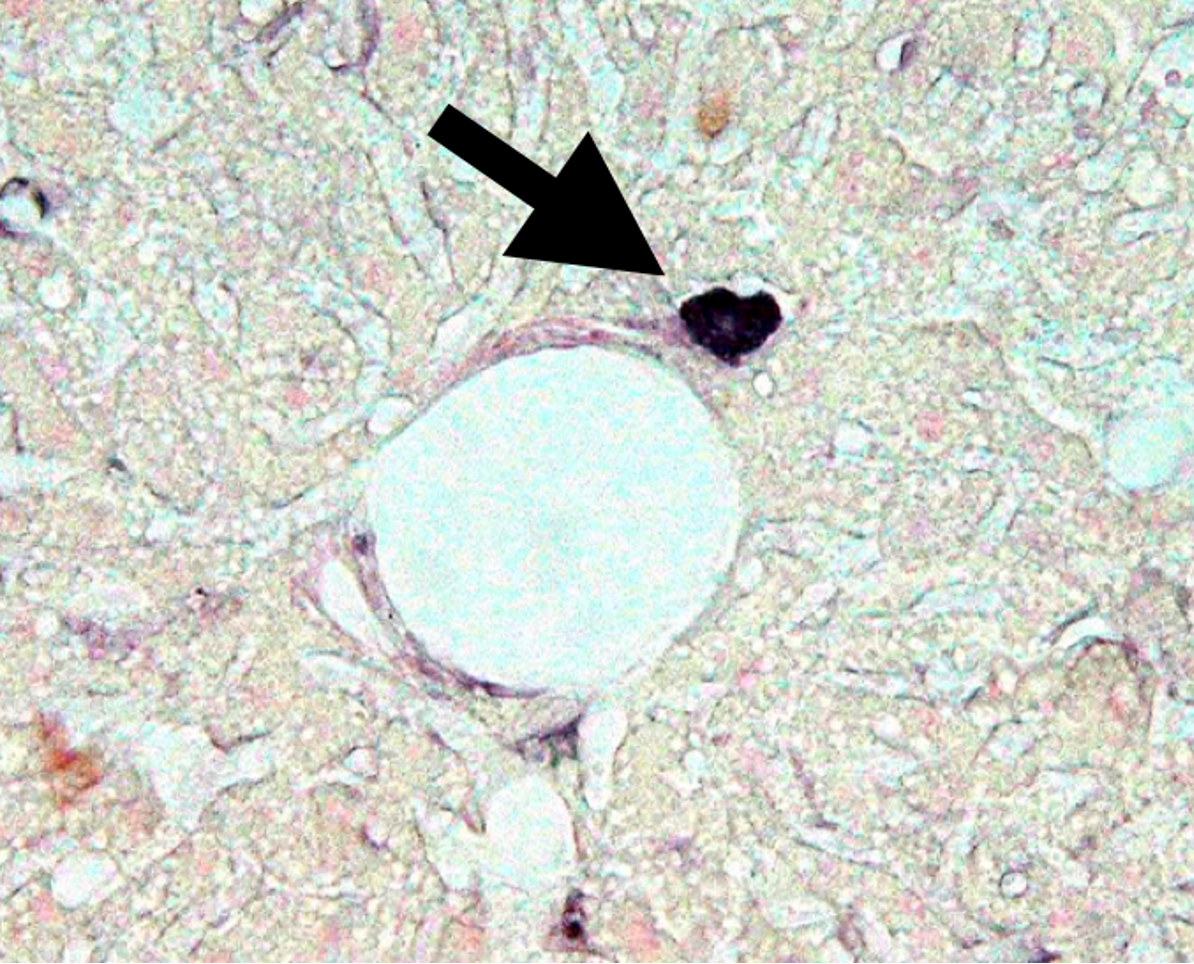
Abnormal stem cells (diabetes stem cells, or DSCs) are a cause of diabetes.
Encounter with Diabetes Stem Cells
The paper “NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice” (Nature Med, 2003) was completed.
This research successfully recreated islets in the liver using NeuroD1. However, one unresolved question remained. Strange cells producing proinsulin were found in the liver of untreated diabetic mice used as controls for gene therapy.
These cells were located right next to the portal vein capillaries in the liver of hyperglycemic mice. In 2003, a paper including microscopic images of these strange proinsulin-positive cells was accepted.
Discovery and Impact of Diabetes Stem Cells
The paper “Complete remission of diabetes with a transient HDAC inhibitor and insulin in streptozotocin mice” (Com Bio, 2023) was completed.
Diabetes is a chronic progressive disease that does not heal naturally. Its cause is linked to genetics, lifestyle, and autoimmunity, but the details are unclear.
Professor Kojima’s team discovered abnormal cells in the hematopoietic stem cell fraction that create a refractory nature for diabetes and its complications. These cells persist even when blood sugar levels are normalized, maintaining their disease stem cell properties.
The removal of these cells, termed “diabetic stem cells,” showed potential for curing diabetes and its complications. Combining insulin with HDAC inhibitors for a certain period removed the “diabetic stem cells,” leading to complete remission of diabetes.
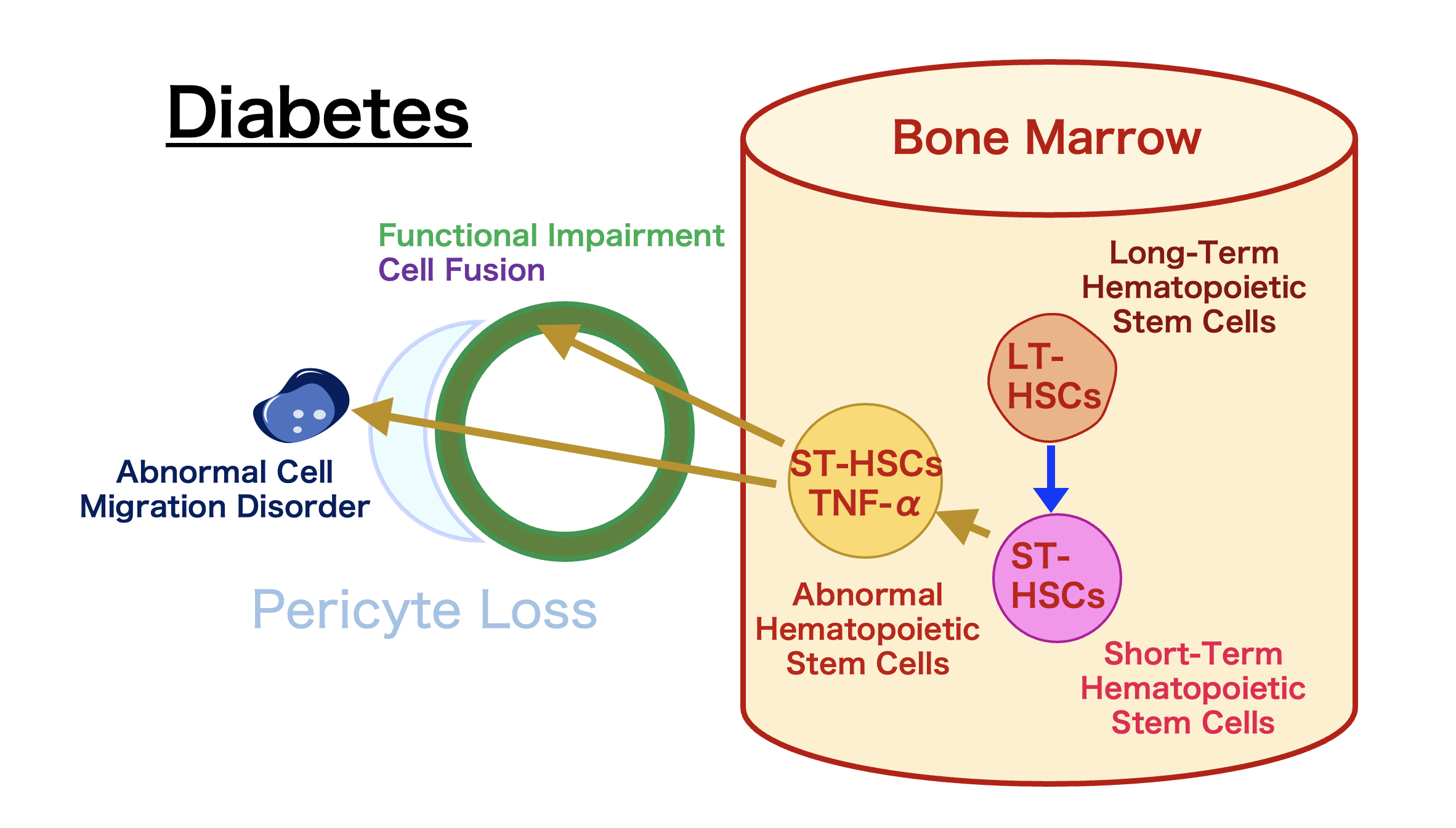
Insulin and Histone Deacetylase (HDAC) Inhibitors
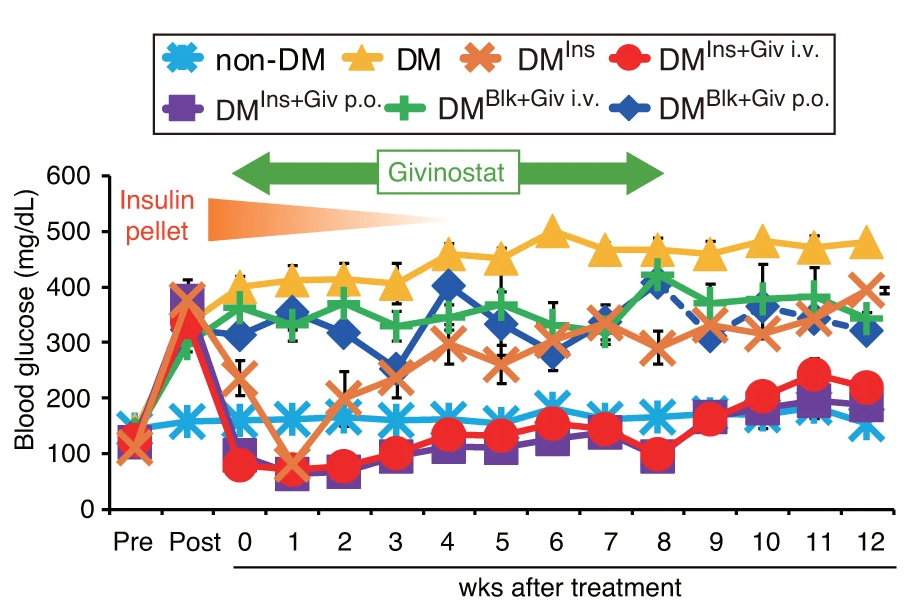
In a study, diabetic mice implanted with insulin pellets were given Givinostat for 8 weeks, which maintained glycemic control and maintained normoglycemia after the insulin pellets were removed, and for an additional 4 weeks after Givinostat treatment was stopped.

Collaboration Research and Joint Venture
KIYAN MEDICAL Co., Ltd. and Biozipcode, Inc. (CEO: Fumihisa Kojima, Advisor: Professor Hideto Kojima) have announced a business partnership for the development of a diabetes cure, new diabetes treatments, and cancer therapeutics. This collaboration is expected to accelerate the development of new drugs.
Starting April 2024, Professor Hideto Kojima will also serve as an academic advisor for KIYAN MEDICAL Co., Ltd., further accelerating research and social implementation involving various companies and universities.
Start in Palau & UAE
Our journey toward a curative diabetes solution begins in Palau, a unique proving ground with both a high prevalence of diabetes and a small, stable population. This environment enables a physician-led clinical trial with 300–500 participants, allowing rapid statistical validation of safety and efficacy. With the Palau National Hospital, volunteer recruitment and ethics approvals move swiftly. Palau’s story mirrors that of Okinawa — a community deeply impacted by diabetes — creating a powerful narrative that attracts global attention and early brand value.
Once validated in Palau, the model expands to the UAE, leveraging Dubai’s position as a hub for medical tourism, regulatory innovation, and Sharia-compliant capital. Through the planned establishment of Biozipcode UAE LLC, the project will serve as the regional headquarters for IP management, a regulatory submission hub for GCC and African markets, and a gateway for capital raising via family offices and sovereign-aligned funds. The goal is to work toward public insurance coverage through the Dubai Health Authority — positioning this as the world’s first curative diabetes therapy eligible for government healthcare reimbursement.



